Introduction
Lithium fluoride is an inorganic salt with chemical formula LiF. It is a colourless solid, that transitions to white with decreasing crystal size, i.e., ground into finer particles at room temperature. Although odorless, lithium fluoride has a bitter-saline taste. LiF is hygroscopic in nature but does not form a hydrate. Naturally occurring lithium fluoride exists as the rare mineral gricite.
Lithium fluoride has a cubic crystalline form, its structure is analogous to that of sodium chloride, but the solubility in water is quite low and chemical reactivity is low. It is insoluble in alcohol and other organic solvents. It is soluble in nitric acid and sulfuric acid, but insoluble in hydrochloric acid and can be dissolved in hydrofluoric acid to generate lithium hydrogen fluoride (LiHF2). At temperatures above 400°C, atmospheric moisture attacks LiF, making it sensitive to thermal shock.
Lithium fluoride is mostly used as the main raw material for production of lithium hexafluorophosphate (LiPF6). Of which is the main chemical salt used to produce electrolyte, a major component material of high-performance lithium-ion batteries. Additionally, other applications for LiF include its use in the ceramics, UV optical, PLED and OLEDs, metallurgy, aluminium and rare earth smelting, molten salts, nuclear reactor, and radiation detection industries.
Production of lithium fluoride can be divided into direct synthesis method, ion exchange method and solvent extraction method.
Lithium Fluoride Characteristics
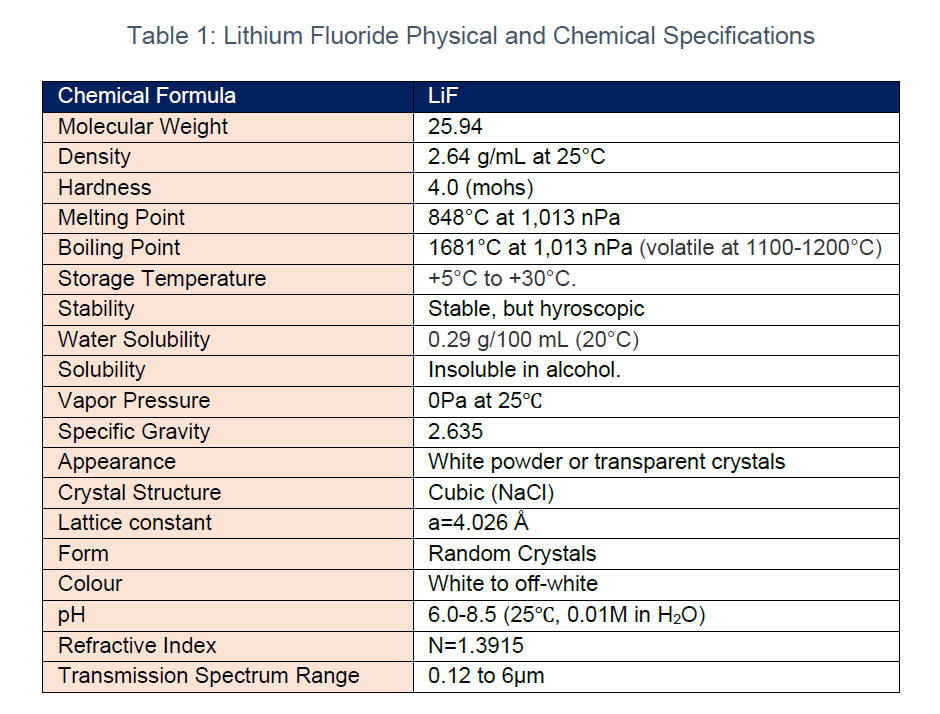
LiF has a molecular weight 25.94, density is 2.640, hardness 4.0, melting point is 848⁰C and boiling point is 1681⁰C. Moreover, it has a wide transmission spectrum from the VUV to IR. In addition, the transmittance of LiF crystal is between vacuum ultraviolet 110nm and infrared 6.0μm, which is the material with the best transmittance in the vacuum ultraviolet region. Lastly, the low index of refraction allows the crystal to be used directly without anti-reflective coating.
Principal Production Process
Lithium fluoride is mostly used as the main raw material for production of lithium hexafluorophosphate (LiPF6). Of which is the main chemical salt used to produce electrolyte, a major component material of high-performance lithium-ion batteries.
Firstly, the production process of lithium hexafluorophosphate involves reacting lithium fluoride with phosphorus pentachloride and hydrogen fluoride. Secondly, the resulting lithium hexafluorophosphate is a white crystalline powder that is thermally stable and highly soluble in polar aprotic solvents. Moreover, these properties make it an ideal electrolyte for lithium-ion batteries. Specifically, the inertness of the hexafluorophosphate anion towards strong reducing agents, such as lithium metal, and its ability to passivate the positive aluminium current collector, are exploited in these applications.
Notably, the main methods to produce lithium hexafluorophosphate include the
- Hydrogen fluoride solvent method
- Organic solvent method
- Gas-solid reaction method
- Ion exchange method
Moreover, the purity of the product obtained by the hydrogen fluoride solvent method is high, thus, it has become the most commonly used synthesis method of lithium hexafluorophosphate in industry.
Commercialisation
The development of electric vehicles has driven the demand for battery-grade electrolyte material. At present, various Chinese companies are accelerating the production output of lithium hexafluorophosphate electrolyte material.
Lithium hexafluorophosphate is a kind of white crystal in solid state or powder with strong deliquescence properties. It is easily soluble in water and soluble in organic solvents such as low concentration methanol, ethanol, acetone and carbonate.
Moreover, Lithium hexafluorophosphate is widely used as electrolyte for lithium-ion batteries, of with characteristics of high safety and ionic conductivity.
Lithium Fluoride Price
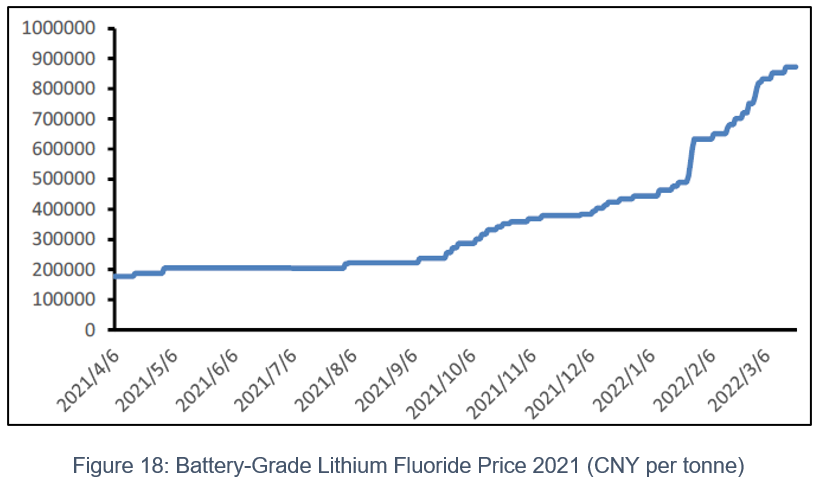
Affected by COVID-19, battery-grade lithium carbonate prices declined in April 2022, dropping to 460,000 CNY/t. While prices for battery grade LiF levelled out at around 800,000 CNY/t. Since August 2022, the demand for downstream procurement and stocking of battery grade lithium carbonate has resumed, and the price of lithium carbonate has risen rapidly. Moreover, this triggered an increase in the price of battery-grade lithium fluoride peaking at around 1,000,000 CNY/t in December 2022.
Contact us to purchase the full report today
Click to view the Table of Contents

What is driving the growth of the lithium fluoride for lithium hexafluorophosphate industry?
Lithium fluoride is essential in the production of lithium hexafluorophosphate, which in turn is a key ingredient in the electrolytes of lithium-ion batteries.
Further reading
Our comprehensive battery mineral research reports include a brief introduction, industry chain, product specifications, processing methods, raw material requirements, cash cost analysis, pricing metrics, future industry development trends, and the competitive landscape. If you have specific reseach topics, reach out by email and contact us today to learn more.
You might be interested in our other battery mineral research reports
For Further Information