Introduction
A lithium-ion battery is a high-performance rechargeable battery that uses lithium ions to store and release electrical energy. A conventional lithium-ion battery has two electrodes, a positive electrode (cathode) and a negative electrode (anode), separated by an electrolyte.
During charging, lithium ions move from the cathode to the anode through the electrolyte and get embedded in the anode. During discharging, lithium ions move from the anode to the cathode through the electrolyte and get embedded in the cathode. The movement of lithium ions between the electrodes is accompanied by the flow of electrons through an external circuit, of which, generates an electric current that can be used to power devices.
The charging and discharging process of lithium-ion batteries is the embedding and de-embedding process of lithium ions. During charging, lithium ions are de-embedded from the cathode and embedded in the anode through the electrolyte. During discharging, the opposite is true, where the lithium ions are embedded in the cathode and de-embedded from the anode.
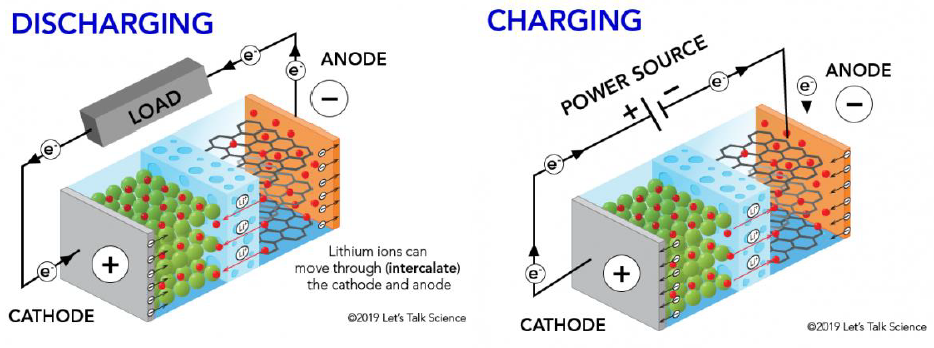
A lithium-ion battery has the characteristics of high energy density, high safety, low self-discharge rate, and long cycle life. Today, lithium-ion batteries are widely used in electric vehicles, energy storage systems, and consumer electronics.

Component Materials
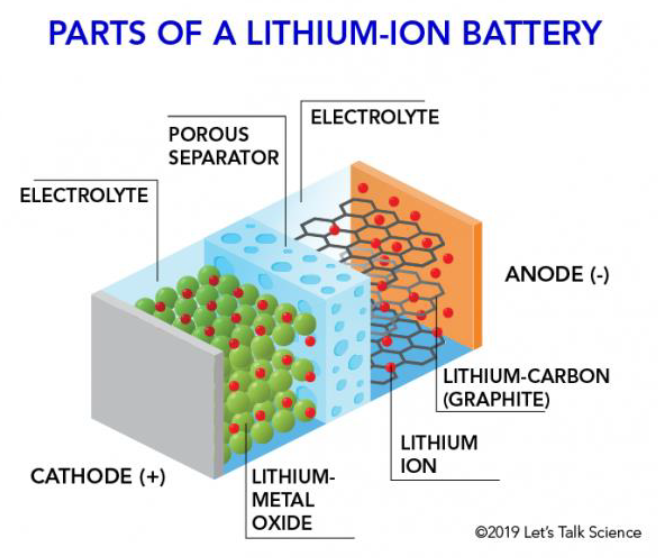
The four major components of a lithium-ion battery include the cathode, anode, separator, and electrolyte:
- Cathode: During discharge, the cathode releases positively charged lithium ions that travel through the electrolyte to the anode. Today, mainstream cathode material products include lithium cobalt oxide, lithium manganese oxide, lithium iron phosphate, and ternary material.
- Anode: During discharge, the anode receives positively charged lithium ions from the cathode, which causes the anode to become negatively charged. The anode is the negative electrode of the battery and is usually made of natural flake graphite or synthetic graphite.
- Separator: The separator is a thin, porous membrane that separates the cathode and anode. This prevents both of them coming into contact with each other allowing lithium ions to move freely between the electrodes. Of which, this is important for lithium-ion battery safety as it prevents short circuits within the cell.
- Electrolyte: The electrolyte is a liquid or gel substance that contains lithium hexafluorophosphate and other solvents. The electrolyte has the function of facilitating the movement of lithium ions between the cathode and anode. During discharge, electrolyte carries positively charged lithium ions from the anode to the cathode and vice versa through the separator.
Packaging Forms
Lithium-ion batteries are available in rigid cylindrical, prismatic (rectangular) and pouch constructions. Cylindrical cells are a mature technology and are used for many battery types. Moreover, prismatic cells have a long history of being used in mobile phones and other consumer electronic devices. Lastly, pouch cells are a new technology, which use a thin, flexible laminate instead of a rigid housing.
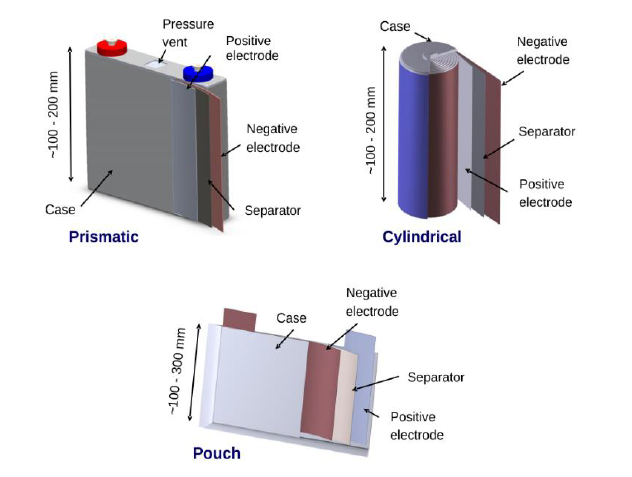
Cylindrical cells are made by rolling long strips of cathode foil, separator, and anode foil together and inserting into a rigid stainless steel or aluminium cell housing. Firstly, the housing is filled with liquid electrolyte, safety disks are inserted into the top. Secondly, the electrodes are welded to the outer battery terminals (in this case, the top and bottom of the cell). Lastly, the cell is hermetically sealed by crimping the top disk assembly closed.
cylindrical cells
Cylindrical cells have high mechanical stability as their shape distributes forces, due to internal pressure increase, evenly over the circumference. However, their shape makes it harder to package them together in an efficient manner. Therefore, a significant amount of space is lost when arranging them in a rectangular shape. However, make it easier for air to flow freely around a group of cylindrical cells resulting in easier thermal management.
Prismatic cells
Prismatic cells use a flat rectangular housing to lower the overall thickness of the cell. The electrode/separator assembly can be rolled, as with cylindrical cells, or it can be a rectangular stack of individual electrodes. Moreover, the battery terminals can be placed as contact pads on the top or side of the housing. The prismatic cell thin form factor is suited for use in consumer electronics, particularly when battery replacement is desirable. Moreover, the prismatic shape makes them easier to integrate in a battery pack than cylindrical cells. However, this can make it more challenging to regulate their temperatures.
Pouch cells
Pouch cells have a thin rectangular form factor. Firstly, they are composed of rectangular stacks of individual electrode/separator layers, but instead of a rigid metal case they use a laminated flexible polymer/aluminium “bag”. Secondly, the electrodes have tabs along one side, these are welded together with battery terminal tabs that stick out of the top of the bag. Lastly, the assembly is saturated with a liquid electrolyte and the bag is heat-sealed.
Manufacturing Costs
In terms of a lithium-ion battery manufacturing cost composition, the material cathode accounts for the largest cost portion, 45% of the total cost, followed by the anode accounting for 10%, separator accounting for 10%, electrolyte accounting for 10%, with the other components materials making up about 25%. As the cathode accounts for the largest cost to produce a battery, it plays a pivotal role in industry development.
Furthermore, by eliminating the rigid housing, pouch cells save on cost, weight and thickness. The flat shape allows for very high packaging efficiency of 90-95% when integrating them in battery packs. The flexible pouch is, however, prone to swelling as it is more difficult to dissipate heat and this can pose problems with lifetime, capacity loss, and safety.
Further reading
What is driving the growth of the global lithium-ion battery industry?
Growth of the global lithium-ion battery industry is driven by the need to reduce climate change, regulatory shifts toward sustainability, and the surging demand for lithium-ion batteries from plug-in vehicles and the renewable energy sector.
How is the market segmented and which countries are leading its growth?
LFP cathode material is mostly produced in China, Japan, and South Korea
Why are LFP cathode material and ternary cathode material becoming popular?
LFP cathode material is mature technology which exhibits high safety, long cycle life, and good energy density. Whereas, ternary cathode material enjoys high-energy density, and long cycle life.
Our research reports include an introduction, industry chain, product specifications, processing methods, raw material requirements, cash cost analysis, pricing metrics, future industry development trends, and the competitive landscape. Reach out by email and contact us today to learn more.
You might be interested in our other battery mineral research reports
For Further Information
